Introduction
Oral cancer is considered to be a serious global public health issue with an estimate of more than 7.6 million deaths annually. In India, the incidence of oral cancer is estimated to be 73.6 per 100,000 populations. The overall mortality rate for oral cancer remains high at approximately 50%, even with recent existing medical facilities.1 Oral cancer is the sixth most frequent cancer worldwide, with a high prevalence in South Asia with estimating about 1/4th of cancers in males and 1/10th of cancers in females in India.2, 3 Oral squamous cell carcinoma (OSCC) is considered to be one of the foremost cancers across the globe. Several studies have been conducted regarding the natural history, epidemiology, and clinicopathological classification of OSCC so far.4 The annual mortality rate from head and neck carcinoma (HNSCC) is over 11,000, relating to 2% of all cancer deaths. HNSCC commonly metastasizes to the regional lymph nodes. The lymph nodes being the first site of blockage for tumor cells that have invaded the peritumoral lymphatics; appears to be the most evident predictor of disease prognosis and consequence.2
The most vital prognostic marker for patients with OSCC is metastasis to cervical lymph nodes or distant organs. Metastasis is a process comprising of progressive and selective stages - proliferation, induction of angiogenesis, detachment, motility, invasion into circulation, aggregation and survival in the circulation, cell arrest in distant capillary beds, and extravasation into organ parenchyma. The progression of metastasis varies according to the interaction between host factors and intrinsic characteristics of cancer cells.5, 6 Micrometastasis refers to the microscopic deposits of malignant cells discrete from the primary lesion. Lymph node metastasis decreases the survival rate by 50%.7, 8 Micrometastasis to the regional cervical lymph nodes is a fundamental prognostic element and significantly essential in survival, recurrence and treatment modality.3
De Masceral et al.(1992) defined micrometastasis as metastatic deposits which measured less than 0.5 mm in diameter.9 The Union for International Cancer Control (UICC) has classified and staged the tumor deposits within the lymph nodes that proposed micrometastasis as metastasis occupying between 0.2mm to 0.2cm of the sectioned area.10 The characteristic isolated tumor cells of the lymph nodes can be detected only by immunohistochemistry and the discovery of isolated (disseminated or circulating) tumor cells should be separated from micrometastasis (occult metastasis). Micrometastasis takes place when there has been blockade and lodgment of tumor cells in the organ involved, extravasations, proliferation and stromal reaction.11, 12
The 5year survival rate of oral carcinoma patients still remains far beneath that of various other cancers, for example breast and colon cancer. However, there are multiple treatment regimens in recent decades that have been introduced and followed to combat the disease.13 The development of an effective treatment program and enhancement of the 5-year survival rate of oral cancer patients thus depends on the understanding the underlying mechanisms of its pathogenesis.
Angiogenesis encompassing migration and proliferation of endothelial cells facilitates tumor growth and metastasis. Angiogenesis (formation of new blood vessels from pre-existing endothelium) aids in supplying proper nutrients and oxygen to the tumor cells. An avascular tumor fails to grow, expand and even metastasize to distant organs without proper nutrient supply. Thus, the degree of angiogenesis proposes a useful indicator for the presence of lymph node metastasis.13
Pathogenesis of Metastasis
The metastatic cascade is sub divided into two main categories:-
Tumor growth and metastasis depends upon angiogenesis, formation of new blood vessels from pre-existing endothelium. Angiogenesis is a multi-step progression including proliferation, migration and differentiation of endothelial cells and is coordinated by maintaining a balance between angiogenic inducers and inhibitors. The prognosis of several types of tumor is however, associated with intratumoral microvessel density.14, 15
Tumor cells have the capability of producing several biologically active angiogenic factors that help in the disease progression.15 Metastasis turns out to be even more intricate due to the occurrence of micrometastasis that ranges between 11 - 44% in T1-T2 clinically negative lymph node patients and has a definite effect on the prognostic consequence of the disease. Due to the micrometastasis, there is a significant decrease in the survival rate despite the presence of the most aggressive treatment modalities.16 Distant metastasis is thought to be the final form of metastasis in OSCC that has been reported to vary between the range of 3-30% prevalence rate.17 The rate of distant metastasis at initial diagnosis of the oral tumors is estimated to be less than 3%.16
Metastasis of oral cancer is a multifaceted process that includes detachment of tumor cells, regulation of cell motility & invasion, proliferation and evasion through the lymphatic system or blood vessels. This occurs due to diminished intercellular adhesion of tumor cells during malignant growth owing to the loss of E-cadherin. Certain proteins such as mesenchymal vimentin and N-cadherin are expressed from the tumor cells that aid in promoting cell elongation and interfering with cell polarity. This morphological transition, known as epithelialmesenchymal transition (EMT) leads to molecular alterations interfering with the nature of these cells.18
The metastatic potential of OSCC is significant during the tenure of selecting appropriate treatment modality, thus compelling aggressive therapeutic modalities to ensure residual disease-free effect. The estimated risk of micrometastasis in early stage cases is around 30% that predominantly increases the risk of recurrence and reduces survival ratio. Neck dissection is proposed for elective treatment and staging for patients with clinically negative lymph node in stages I and II. However, the indications and benefits of this method in the head and neck area are still debatable. Numerous retrospective studies have been conducted to recognize the advantages of the technique.16
Grading and Staging of Metastasis
Tumor cell deposits and micrometastasis within the lymph nodes were classified and staged according to the revised guidelines which were set by the UICC (International Union Against Cancer): metastasis ≥2 mm, micrometastasis >0.2 and <2 mm, and isolated tumor cells ≤0.2 mm. The detection of micrometastasis has resulted in an “upstaging of tumors” and the incorporation of micrometastatic disease into the TNM staging system. The UICC/TNM protocols have been established for head and neck sentinel nodes.19
Generic TNM coding for sentinel nodes:
pNX (sn) Sentinel lymph node could not be assessed
pN0 (sn) No sentinel node metastasis
pN1(sn) Sentinel node metastasis.
Sentinel nodes with micrometastasis only are identified by (mi):
pN1 (sn) (mi) Single ipsilateral node with micrometastasis
pN2 (sn) (mi) Multiple ipsilateral nodes with micrometastasis.
Sentinel nodes with isolated tumor cells are coded separately for morphological and nonmorphological techniques such as PCR or flow cytometry:
pN0 (i‑)(sn) No sentinel lymph node metastasis histologically, negative morphological findings for isolated tumor cells (ITC).
pN0 (i+)(sn) No sentinel lymph node metastasis histologically, positive morphological findings for isolated tumor cells (ITC).
pN0 (mol‑)(sn) No sentinel lymph node metastasis histologically, negative nonmorphological findings for isolated tumor cells (ITC).
• pN0 (mol+)(sn) No sentinel lymph node metastasis histologically, positive non-morphological findings for is Regional metastasis to the lymph nodes had a definite bearing on prognosis. Any proposal to treat micrometastasis must first clarify which detection methods should be used to identify these malignant cell isolated tumor cells (ITC). 8
Detection of Micrometastasis
Oral cancer spread through lymphatic pathways to the cervical lymph nodes making it prognostic marker for epithelial malignancies and rules the indications for adjuvant therapy.(20) Early recognition of micrometastatsis helps to detect the patients who would most possibly benefit from adjuvant therapy and also aid in surgical planning and prognosis of the disease.20, 21
There are several sensitive techniques for detection of micrometastasis including Immunohistochemistry (IHC), Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) and serial sectioning. However, these methods have many disadvantages wherein both IHC and RT-PCR are expensive and technique sensitive. Hence, it is basically not feasible for the surgeon to subject several nodes to detect micrometastasis. Serial sectioning, likewise, has the disadvantage of being very lengthy, labor and time consuming. Therefore, detection of micrometastasis by a method which is easily feasible and inexpensive is quiet essential for the benefit of the patients.7
There are certain special stains like Modified Papanicolaou stain and Toluidine blue that are readily available and can be used on serial sectioned lymph node sections to detect micrometastasis which are usually overlooked in single-section Hematoxylin and Eosin stain.3 Thus, these special stains stain are certainly valuable and sensitive in detecting micrometastasis by serial sectioning technique. Detection of micrometastasis in OSCC patients especially after surgery is undoubtedly beneficial for the patient as aids in modifying the treatment protocol inclusive of both radiotherapy and chemotherapy.3
Methods of Detecting Micrometastasis
The different methods of detection of micrometastses are sentinel lymph node biopsy, immunohistochemistry, molecular detection and detection of micrometastasis in bone marrow and blood.
Sentinal lymph node biopsy (SLN)
Primary tumor drains first into the lymph node which are referred to as SLN and the primary afferent lymphatic first drains into the SLN of the particular basin.22 The first node that is reached by the lymphatic stream are the SLN denoting an systematic and consecutive drainage from the tumor site. This also depicts the nodal stage for accurate histological staging of the disease.23 The technique entails the usage of injecting a radiolabelled colloid around the primary tumor that drains into the first level lymph nodes, and this can be detected by the use of gamma probes.8
Advantages of sentinel node biopsy:
Pathological examination for micrometastatic deposits for small volume of tissue can be done in details.
Morbidity of nodal acquisition is reduced and it may obviate formal lymph node dissection in patients with negative sentinel nodes.
Metastatic cells can be accurately identified through multiple sections of nodes.
The Sentinel Node European Trail (SENT) pathology protocol has recommended evaluation of the SLN biopsy that has the ability to detect all micrometastatic deposits with assurance.8
Figure 1
Overview of the metastatic cascade: The five key steps of metastasis include invasion,intravasation, circulation, extravasation, and colonization24
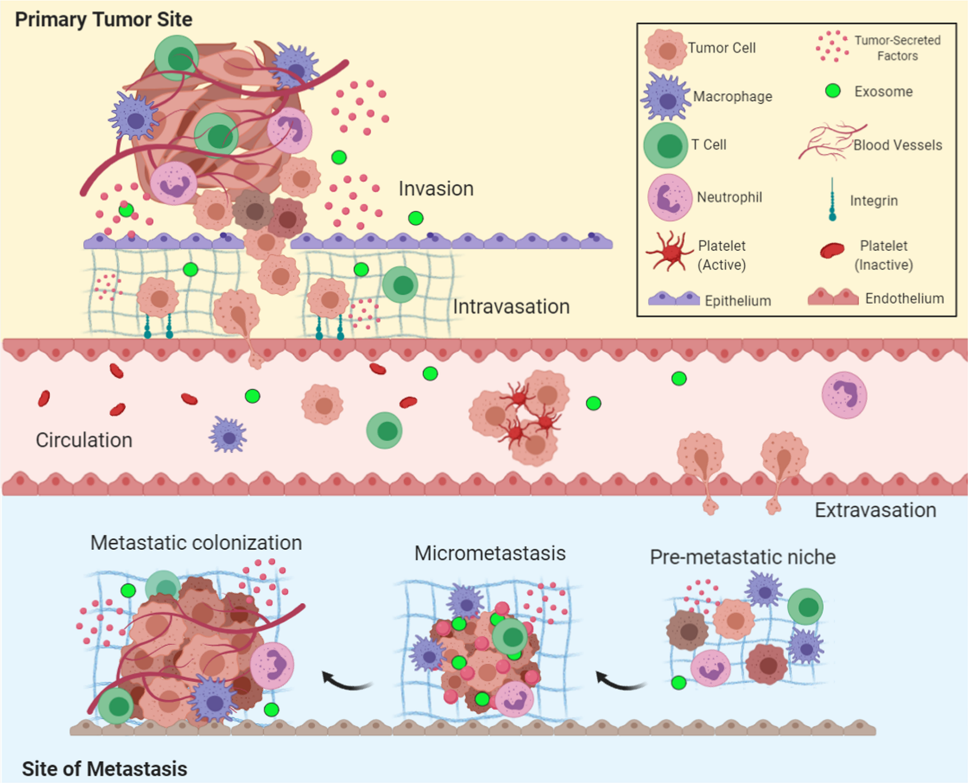
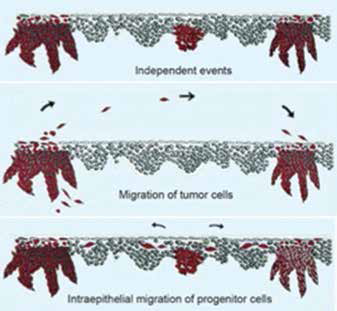
Figure 2
Mechanisms of oral fieldcancerization showing (a) independent events, (b) migration of tumor cells, and (c) intraepithelial migration of progenitor cells25
Immunohistochemistry
Cytokeratin proteins form a crucial constituent of the cytoskeleton of the epithelium and function as reliable markers for identifying micrometastasis. The epithelial cells in the lymph nodes may be detected by the use of specific anti‑cytokeratin (CK) antibodies against these cytokeratin proteins. CKs are extremely expressed in epithelial tumors and thus, CK19 and CK20 have been evaluated frequently. Germani et al.(2009) performed a study to identify Membrane Type‑1 Matrix Metalloproteinase (MT1‑MMP) by immunohistochemical analysis in OSCC and concluded that MT1‑MMP positivity is suggestive of aggressive tumor types, verified due to the presence of micrometastasis.8 Barrera, et al. (2003) studied the incidence of occult nodal metastases with HNSCC and the clinical significance of nodal micrometastasis by immunohistochemical (IHC) staining of cytokeratins.26 The frequency of distant metastases rises with increasing stage of the disease. In 1997, Byers discovered the lymphatic drainage patterns of the tongue being responsible for higher chances of skip metastasis. It was thus deduced that, skip metastasis presented a high rate of reoccurrence of tumor with decreased survival.22
Molecular methods based on the amplification of tumor cell DNA or complementary DNA reverse transcribed from mRNA by the polymerase chain reaction (PCR) have been used to detect tumor cells in lymph nodes. However, the specificity of RNA-based markers (CEA) the mRNA, recently utilized for the analysis of lymph nodes in patients with colon cancer, is not appropriate due to the low-level illicit expression of the marker gene in the surrounding lymph node cells. There are better alternatives like DNA based markers, such as mutations in the p53 gene or Ki-ras gene which have been used in patients with colorectal cancer, lung cancer, or head and neck cancer to detect single tumor cells in a background of thousands of lymph node cells.20
Molecular detection
Molecular assays are extremely sensitive as compared to standard histopathological methods. Brenan et al. originally used PCR based assays with p53 gene for detection of micrometastasis in head and neck cancer. They found that the number of nodes that showed evidences of micrometastasis was much higher in molecular studies as compared to light microscopic study.27
PCR is an in vitro process of amplification of specific DNA sequences with the help of oligonucleotide primers (short DNA sequences) and therefore define the region of interest in the target DNA. The procedure comprises of a series of cycles, consisting of template denaturation, primer annealing, and extension of the annealed primers by a thermostable DNA polymerase to create the exponential accumulation of a specific DNA fragment. The ends of these cycles are distinguished by the 5’ ends of the primers. After about 20 cycles, the amplification is about hundred fold. PCR amplification is also done using RNA as starting material, known as RT-PCR. This method is analogous to DNA PCR with the modification that PCR amplification is headed by reverse transcription of RNA into cDNA.28
These assays however can reliably assess relapse and death and would likely have a major influence on the treatment of many solid tumors. These methods can also improve the preoperative staging of patients with epithelial malignancies thus avoiding needless radical procedures. Additionally, these procedures aids in monitoring the efficiency of various adjuvant therapy.
Detection of micrometastasis in bone marrow and blood
Oral cancer has high recurrence rate owing to the presence of numerous tumor cells that are dispersed and identified in the hematopoietic cell compartment. There are several practices presently used for detection of these isolated tumor cells that depend on examination of monocytes derived from peripheral or central venous blood or bone marrow. The common methods utilized for detection are immunocytochemistry, flow cytometry and RT‑PCR.8
Conclusion
It is documented that histological tumor differentiation and lymph node metastasis serves to be good predictors when designing therapeutic approaches for OSCC. Hence, it is advisable to assess the potential biological characteristics and deliver necessary information about the nature of the tumor preoperatively. Thus, the choice of treatment protocol highly depends on these diagnostic aids to clearly delineate the level of the cervical metastasis. The management of OSCC patients with metastasis, or those with high risk of metastasis, needs invasive management methodologies to guarantee the finest result.
Micrometastasis is considered to be the utmost prime killer of the OSCC patients. Molecular diagnostics techniques have revealed higher than ever metastatic rates, up to 30%, (in very early stages). Some authors also believe that that metastasis originates as early as the tumor itself. Thus, understanding the molecular mechanism of the pathogenesis of metastasis, helps to establish the foundation for precise clinical models. This will ensure the identification of the critical characteristic of those cells that are crucial for development of effective therapeutic modalities.
Although, clinical oncology has become extremely advanced, the existence of micrometastasis has restricted improvements in lethality rates. Thus, it can be concluded by saying that since lymph node micrometastasis appears to be an essential adverse prognostic factor in oral and oropharyngeal SCC, it becomes the utmost responsibility of the clinician to inculcate various methods for the detection of micrometastasis into future clinical trials and management strategies for better understanding and outcome of the disease.
