Introduction
Periodontitis is progressive inflammatory disease that cause loss of support for the affected teeth, especially the fibers of the periodontal ligament and the bone in which they are placed. The expected curative effect of periodontal treatment is regeneration or repair. This is determined by two critical events: the availability of the required cell types and the presence or absence of the appropriate signals to attract and activate cells. The development of the clot initiates the healing cascade of any wound, which is then followed by the phases of proliferation and maturation. Growth factors promote wound healing by promoting cell proliferation (biogenesis), cell migration (chemotaxis) and the creation of new blood vessels (angiogenesis).1 The use of blood-derived products for the treatment of wounds began in 1970 with the introduction of fibrin glues, produced by the polymerization of fibrinogen with thrombi and calcium.2, 3 Fibrin glue has therapeutic uses such as topical hemostasis and sealing of tissue, soft tissue, and fusion agents for bone particle replacements.4, 5 It has a diameter of 2-3 µm and the cell organelles consist of granules, a few mitochondria and obvious membrane features. It features a surface-connected canalicular system and a well-stacked tubular system that aids in the expulsion of growth factors, especially PDGF, when platelets are activated (Figure 1).,6 Granules are round or oval membrane entities that contain cytoplasm. The diameters of the granules vary between 200 and 500 nm. These macromolecules represent 15% of the total volume of platelets7 and serve as a benchmark for proteins involved in various stages of wound healing, including PDGF, TGF and insulin-like growth factor (IGFI).8 Along with TGF, PDGF stimulates protein synthesis in bone tissue and the proliferative stage of wound healing. TGF stimulates osteoblast and endothelial cells while inhibiting osteoclasts and initiating the growth of intertwined bones. IGF1 promotes the proliferation of osteoblasts and enhances the expression of osteocalcin for matrix formation. A combination of IGF1 and PDGF assesses the rate and quality of wound healing (Figure 2).9
Figure 2
Growth factors released from α granules and their function (Courtesy: researchgate.net). BMP = bone morphogenic protein, VEGF = vascular endothelial growth factor, PGDF = platelet-derived growth factor, TGF-β = transforming growth factor
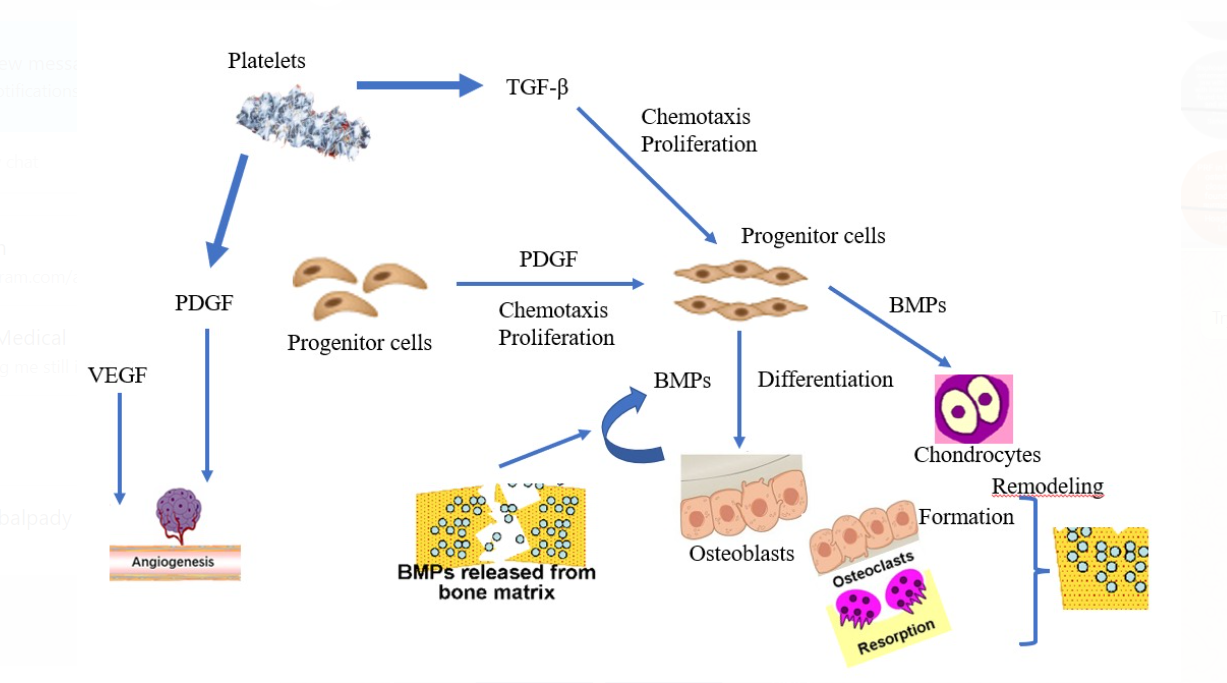
The granules merge with the cell membrane following activation. When activated, the proteins are released, attach to the target cells' transmembrane receptors, activate intracellular signal proteins, and affect the expression of a gene sequence, encouraging healing.
Classification of Platelet Concentrates (Figure 3)
Platelet-rich plasma (PRP), the first-generation platelet concentrate, had good outcomes; nevertheless, its drawbacks included a complicated preparation process and the danger of cross infection from bovine thrombin.
Platelet-rich fibrin (PRF), also known as Choukroun's platelet-rich fibrin after its discoverer.
Platelet-Rich Plasma (PRP)
It is a first-generation platelet concentrate with a high platelet concentration but a low quantity of natural fibrinogen. Within 3–5 days following platelet activation, the granules release growth factors that continue to stimulate the proliferative phase for 10 days.
Obtaining Platelet-Rich Plasma
PRP is derived from autologous blood. The cell separator withdraws 400–450 mL of autologous blood using a central venous catheter. Before centrifugation, 1:5 sodium citrate dextrose is given to the blood for anticoagulation via calcium binding, followed by a two spin centrifugation.
The tube is centrifuged for 10 minutes at 1300 rpm (soft spin). A second centrifugation at 2000 rpm for 10 minutes is conducted (hard spin). After 10 minutes of centrifugation, three layers are produced. The least dense layer, platelet-poor plasma, accounts for about 45 percent of the sample; the intermediate layer, RBCs, accounts for about 40 percent of the sample; and the bottom layer, PRP, accounts for about 15 percent of the sample. It is also known as the buffy coat due to its white or buffy look.
Each combination contains 7 mL PRP, 1 mL CaCl2, 1000 units of topical bovine thrombin, and 1 mL air (the CaCl2 and thrombin initiate the coagulation process and easy handling). It is then agitated for 6–10 seconds to promote clotting before being applied to the graft site or mixed with particle graft. It can also be gelled and applied to a surgical site as a membrane.
PRP architecture consists of bilateral junctions (condensed tetra molecular) formed by high thrombin concentrations; fibrin polymer thickening, producing a stiff network adverse to cytokine enmeshment; and cellular movement.10 Periodontal defects, root covering treatments, ridge augmentation grafting, guided bone regeneration, sinus lift grafting, and implant surgery are all periodontal uses of PRP. Mandibular and maxillary repair (tumour and trauma-related deformities), blepharoplasty, cutaneous fat grafts, and orthopaedic surgery are also therapeutic applications of PRP.
The following are some of the benefits of utilising PRP: delivers cytokines and growth factors to the region, which aids in fast regeneration in a way that fibrin glue would not because of the presence of platelets; free of worries about transmissible illness; and convenient for the patient.
However, its drawbacks include a lack of uniformity in the PRP preparation procedure, variations in the storage period of various platelet concentrations, and the risk of life-threatening coagulopathies (bovine thrombin can trigger antibodies to clotting factors).
Plachokova et al.11 discovered evidence for PRP's positive benefits in the therapy of periodontal abnormalities in a recent systematic study. The evidence for the positive benefits of PRP in sinus elevation appeared to be minimal, and no conclusions could be reached about its other dental uses.
In the intervening time, Kotsovilis et al.12 observed varying results regarding the effectiveness of PRP with various therapeutic bioactive agents/procedures, potentially implying that the precise selection of agents/procedures combined with PRP is crucial. According to Bae et al.,13 there is enough data to justify the use of PRP for bone growth on a sinus bone transplant, although its influence on implant longevity is less substantial.
Platelet-Rich Fibrin
It is a kind of fibrin that is rich in platelets. Choukroun et al.14 (2001) created an autogenous live biomaterial that is a second-generation platelet concentrate in France. It has grown in popularity due to its ability to speed up soft- and hard-tissue recovery. Its advantages of PRP include simplicity of preparation/application, low cost, and the absence of biochemical alteration (no bovine thrombin or anticoagulant is required).14 The fact that PRF has a straightforward preparation process is a significant benefit. The difference between a natural blood clot and a PRF is that the latter is more homogenous and stable, as well as easier to handle and install.15
Procedure for Preparing Platelet-rich Fibrin
The technique attempts to collect platelets and the cytokines produced in a fibrin clot.16 Only centrifuged blood, free of anticoagulants and bovine thrombin, is required for the production of PRF. Blood samples are collected in 10-mL glass or glass-coated plastic tubes without anticoagulant and promptly centrifuged at 3000 rpm for 10 minutes.17 The end result has three layers: The top layer is an acellular plasma, followed by a PRF clot in the centre and a red corpuscle base at the bottom (Figure 4).18
Mazor et al.8 proposed that clots might be converted into membranes by compressing them between two sterile gauzes or in a specialised PRF instrument. When blood comes into touch with a silica surface, the clot polymerization process is activated; this activation decreases the
When compared to the use of bovine thrombin for PRP preparation, there is a higher risk of cytotoxicity.19 In comparison to PRP, it has equilateral connections (connected trimolecular), a thin and flexible fibrin network, and cytokines enmeshment and cellular mobility. The three-dimensional structure of the PRF membrane provides its elasticity, flexibility, and strength.20
The advantages of PRF as a bioactive substitute include less technical skills, minimal biochemical change, cost effectiveness, better integration of circulating cytokines in fibrin meshes, and delayed polymerization, which speeds up healing and enhances structural integrity.21
PRF's stated restrictions include its limited quantity and the necessity for immediate use after preparation since shrinkage due to dehydration may result in structural integrity loss. 19The presence of leukocytes alters the biologic properties of the material, and bacterial contamination occurs during storage.22
Several authors have proposed specific therapeutic uses of PRF as a viable therapy option for tissue regeneration (Figure 5). Simonpieri et al.15 developed the concept of "natural bone regeneration," which entails rebuilding the whole alveolar bone as well as restoring gingival volume and peri-implant bone.
Chang and Zhao23 found that PRF treatment for periodontal infrabony anomalies resulted with good clinical results.Clinical experiments on maxillary sinus floor augmentation and sinus membrane perforation yielded positive results24 The combination of PRF and bone transplant reduces the quantity of bone replacement while the angiogenesis feature improves revascularization. Simonpieri et al.15 saw good clinical results when several layers of PRF were used with rapid implantation.
According to the literature, some other possible applications of osteitis reduction have been discovered in surgical sites of third molars,25 as an adjunct to wound healing of the donor site of free gingival graft,26 in pulp revascularization procedures of immature permanent teeth, and reconstruction after cancer surgery.27 For larger operations, specialised multiple centrifuge tubes can be utilised to produce large amounts of L-PRF.28
The new enhanced PRF generates more growth factor, resulting in the buildup of large quantities of proteins over time.29 According to Gassling et al.,28 PRF appears to be more effective than collagen as a scaffold for cell proliferation, and PRF membranes can be utilised for in vitro growth of periosteal cells for bone tissue engineering.30
Conclusion
PRF, a new type of platelet concentrate, is a game-changing move in regenerative periodontal treatment that requires no metabolic changes and is processed fast. PRF has been used in a number of medical fields, including orthopaedics, plastic surgery, and dentistry. Despite the fact that multiple systematic reviews and meta-analyses have confirmed the advantages and downsides of PRF, additional prospective studies have to be conducted. Clinical trials in cooperative PRF for various treatments are encouraging; nevertheless, further research is needed to enable its broad use in routine dentistry practise with high clinical efficacy and long-term stability.




